Navigation » List of Schools, Subjects, and Courses » Chemistry 51 – Fundamentals of Chemistry » Discussions » Organic Chemistry – Discussion Part 2 » Organic Chemistry – Discussion Part 2 Sample Answers

Organic Chemistry – Discussion Part 2
Representing Organic Compounds
Organic compounds range in size from a few atoms to thousands of atoms. In the complexity of these organic compounds, there are some that have the same chemical formula, but have very different structures. In order to better understand these compounds, scientists have designated the expanded formula, condensed formula, and skeletal structure to represent organic compounds. Please answer the following questions in regards to the representation of organic compounds.
- Identify the differences between the expanded formula, condensed formula, and skeletal structures for organic compounds.
- Based on your understanding of these representations, name the following compounds using the systematic naming scheme for organic compounds:
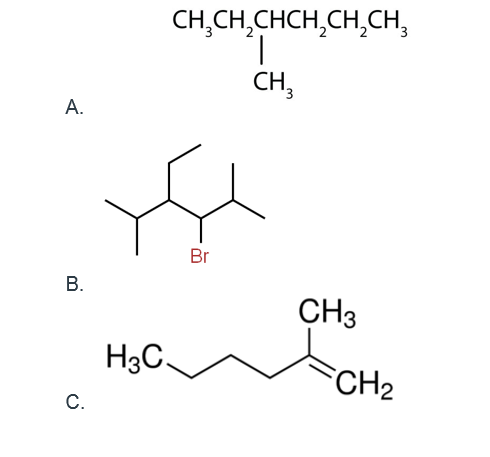
Organic Chemistry – Discussion Part 2
- Identify the differences between the expanded formula, condensed formula, and skeletal structures for organic compounds.
The expanded formula uses lines to represent the bond between atoms. A single line means a single bond; two line, a double bond; and three lines, a triple bond. It can show the branching and the bonding of the atoms. In the condensed form, no lines are used and the user is assumed to have knowledge of the maximum number of bonds an atom can have.

